Si vous êtes à la recherche de Template – European Medicines Agency vous êtes came à droite web. Nous avons 24 paper à propos de Template – European Medicines Agency comme Vos courriels souffrent-ils d’infobésité ? – Société d’histoire et, Guideline on Active Substance Master File · PDF fileGuideline on Active et aussi Quality of Substances for Pharmaceutical Use The EDQM. Lire la suite:
Template – European Medicines Agency

studylib.net
medicines substance
Submitting Electronic Drug Master Files (DMF) And Active Substance Ma…

www.slideshare.net
substance asmf dmf submitting
Quality Of Substances For Pharmaceutical Use The EDQM
present5.com
edqm substances pharmaceutical quality use advertisements substance active master file
PPT – EU Requirements For Quality Of APIs In Marketing Authorisation

www.slideserve.com
developments substance active master file authorisation apis requirements application marketing eu latest quality asmf presentation ppt powerpoint
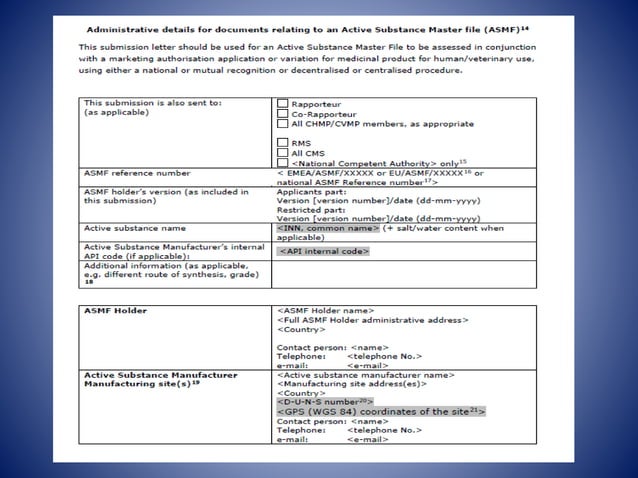
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT
www.slideshare.net
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA

www.slideshare.net
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT

www.slideshare.net
Difference European Drug Master File & Us Drug Master File

www.slideshare.net
difference dmf
CEP & ASMF – NUVOconsultancy

nuvoconsultancy.com
asmf cep dmf
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT

www.slideshare.net
2022.03.24 – 欧盟原料药ASMF注册管理制度简介_科威利华_国产进口药品注册_药品美国欧洲注册_中国国际gmp咨询

cowaymed.com
Guideline On Active Substance Master File · PDF FileGuideline On Active

vdocument.in
PPT – Saravanaraja Subramanian PowerPoint Presentation, Free Download

www.slideserve.com
substance active master file asmf subramanian europe presentation ppt powerpoint
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT

www.slideshare.net
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA

www.slideshare.net
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT

www.slideshare.net
Drug Master File – DMFs – Seedling RA Solutions

seedlingrasolutions.com
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT

www.slideshare.net
(PDF) Guideline On Active Substance Master File Procedure€¦ · 3

dokumen.tips
Vos Courriels Souffrent-ils D’infobésité ? – Société D’histoire Et

histoiregenealogie.ca
Drug Master File

www.slideshare.net
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA

www.slideshare.net
(or The "European Active Substance Master File (ASMF)")

studylib.net
ACTIVE SUBSTANCE MSTER FILE (ASMF) EMA | PPT

www.slideshare.net
Active substance mster file (asmf) ema. Active substance mster file (asmf) ema. Vos courriels souffrent-ils d’infobésité ?
